Local Tissue Regenerative Medicine
1. Tissue regenerative therapy
The tissue regenerative therapy is drawing a lot of attention as a new direction for drug discovery with the goal of regenerating tissues and organs that are functionally damaged due to disease, injury or aging. There are many therapies based on proteins and genes (DNA and viral vectors) as a modality of regenerative medicine are under investigation. However, in reality, the development of these therapies has not progressed sufficiently.
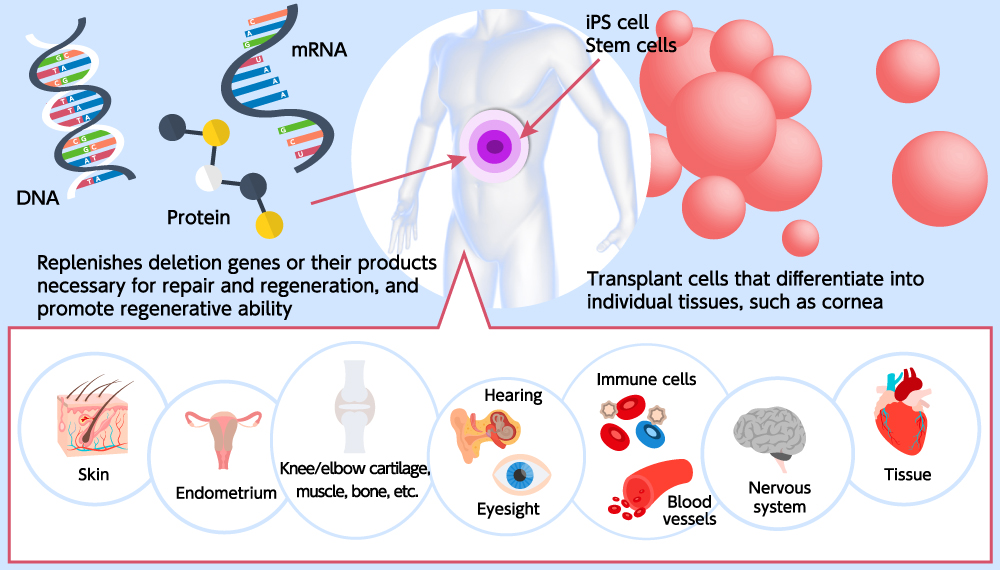
2. Local tissue regeneration by mRNA therapeutics
This mRNA medicine is an application of messenger RNA, which is a short-term blueprint for translating the genetic information in genome DNA into proteins. mRNA medicine has become high-profile because of the COVID-19 mRNA vaccines, and it has been highly regarded worldwide, being the subject of the 2023 Nobel Prize in Medicine or Physiology.
The Nobel Committee praised the technology for converting the natural-type nucleic acids that constitute mRNA into modified nucleic acids. The COVID-19 vaccines ensure a significant reduction of immunogenicity and a drastic improvement in translation into proteins by converting uridine into 1-methyl-pseudouridine. It is no exaggeration to say that with this technology, the potential of mRNA therapeutics and mRNA vaccines is infinite. Now, there are many ongoing projects developing mRNA therapeutics in a wide range of fields.
In 2013, Zangi and his colleagues from Harvard University reported that the administration of mRNA of vascular endothelial growth factor (VEGF) or the above mentioned modified RNA directly into the heart promoted the differentiation and proliferation of the myocardial progenitor cells of mice, restoring cardiac function and showing a life-extending effect.
They proved that gene therapy exacerbated clinical conditions because of the excessive action of VEGF, and mRNA obviously excelled in its therapeutic effects. Then, AstraZeneca and Moderna Therapeutics jointly developed AZD-8601 based on VEGF modified mRNA. Having confirmed safety in a first-in-human phase I study in patients with type 2 diabetes, they conducted a phase II study in patients with myocardial infarction undergoing coronary artery bypass surgery. They reported that the study found a tendency toward the improvement of cardiac output without any major adverse reactions as a result of the direct application of mRNA. This means that they achieved a proof of concept (POC) in applications for mRNA therapeutics for regenerative medicine, a world first.
Unfortunately, they decided to discontinue the clinical development of AZD-8601 in 2023. Instead of AZD-8601, however, Moderna Therapeutics is carrying out the non-clinical development of relaxin (mRNA-1804) formulated using lipid nanoparticles (LNPs) for acute heart failure, preparing for a phase 1 study. It was reported in 2019 that the recombinant relaxin protein Serelaxin (Novartis) did not show significant improvement of heart failure in a phase 3 randomized study with 6500 subjects.
The following lists the companies conducting the clinical development of mRNA therapeutics in regenerative medicine, target molecules, drug delivery systems (DDSs), diseases and other information.
